
ICH Guidelines for Pharmacovigilance
ICH Guidelines. All Guidelines; Quality Guidelines; Safety Guidelines; Efficacy Guidelines; Multidisciplinary Guidelines; Index of Guidelines; ICH Standards. MedDRA; CTD; Electronic Standards (ESTRI) Other Work Products. Reflection Papers & Discussion Groups; Consideration Documents; CIOMS Glossary of ICH Terms & Definitions; Meetings .

ICH pharmacovigilance planning, an efficacy guideline
1.1 Objective. This guideline is intended to aid in planning pharmacovigilance activities, especially in preparation for the early postmarketing period of a new drug (in this guideline, the term "drug" denotes chemical entities, biotechnology-derived products, and vaccines).

ICH Guidelines for Pharmacovigilance
You can explore in the below table the index of all ICH Guidelines, finalised or under development, on the topics of Quality, Safety, Efficacy and Multidisciplinary. Please select first the relevant topic. You can then search by ICH Step status, date, and/or by keyword.

The Importance of ICH GCP CCRPS
This document provides guidance on planning pharmacovigilance activities, especially in preparation for the early postmarketing period of a new medicinal product. It applies to chemical entities, biotechnology -derived products and vaccines.

ICH GCP
This International Conference on Harmonization (ICH) guidance addresses the choice of control group in clinical trials, discussing five principal types of controls, two important purposes of.

ICH Guidelines for Pharmacovigilance
Efficacy Guidelines The work carried out by ICH under the Efficacy heading is concerned with the design, conduct, safety and reporting of clinical trials. It also covers novel types of medicines derived from biotechnological processes and the use of pharmacogenetics/genomics techniques to produce better targeted medicines.

ICH pharmacovigilance planning, an efficacy guideline
Good pharmacovigilance practices (GVP) are a set of measures drawn up to facilitate the performance of pharmacovigilance in the European Union (EU). GVP apply to marketing-authorisation holders, the European Medicines Agency (EMA) and medicines regulatory authorities in EU Member States.

ICH Guidelines for Pharmacovigilance
E6 (R2) Good clinical practice. - Scientific. guideline. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects. It aims to provide a unified standard for the ICH regions to facilitate.
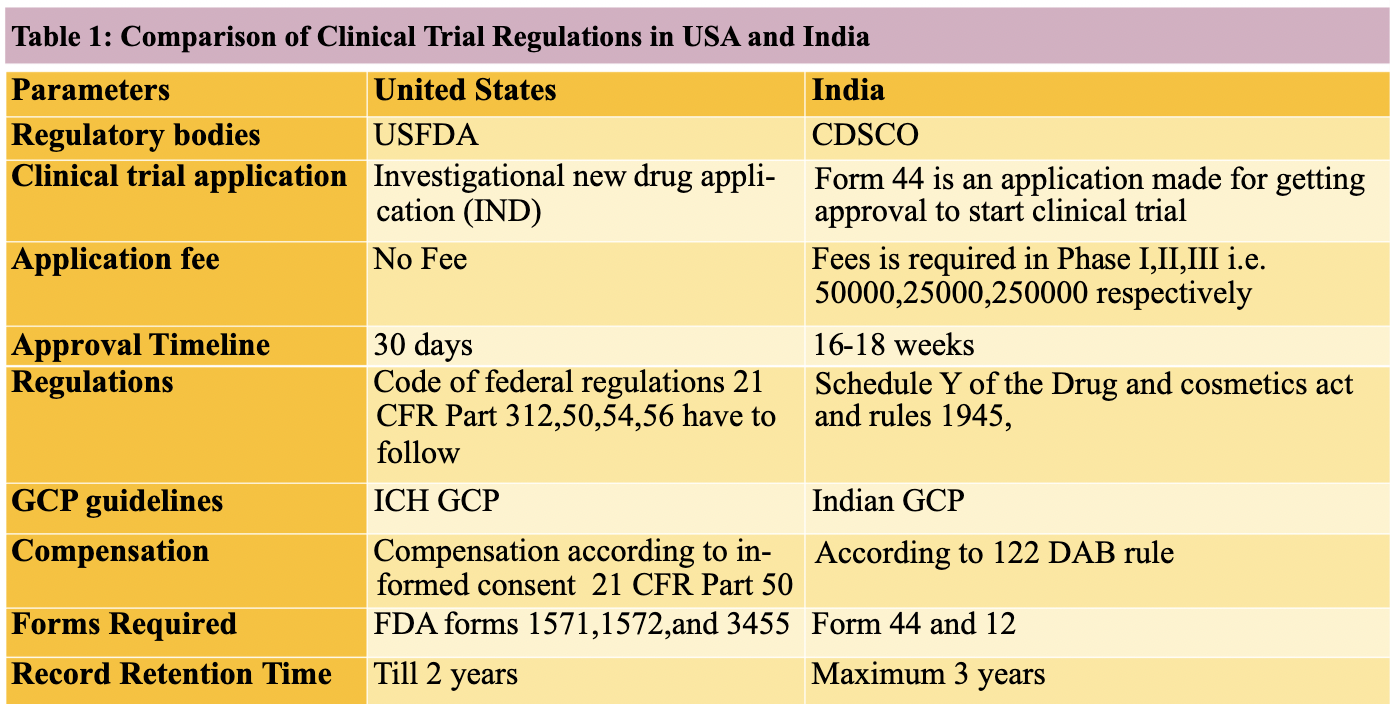
Difference between ICH GCP and schedule Y CCRPS
INTEGRATED ADDENDUM TO ICH E6(R1): GUIDELINE FOR GOOD CLINICAL PRACTICE ICH E6(R2) INTRODUCTION Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects.
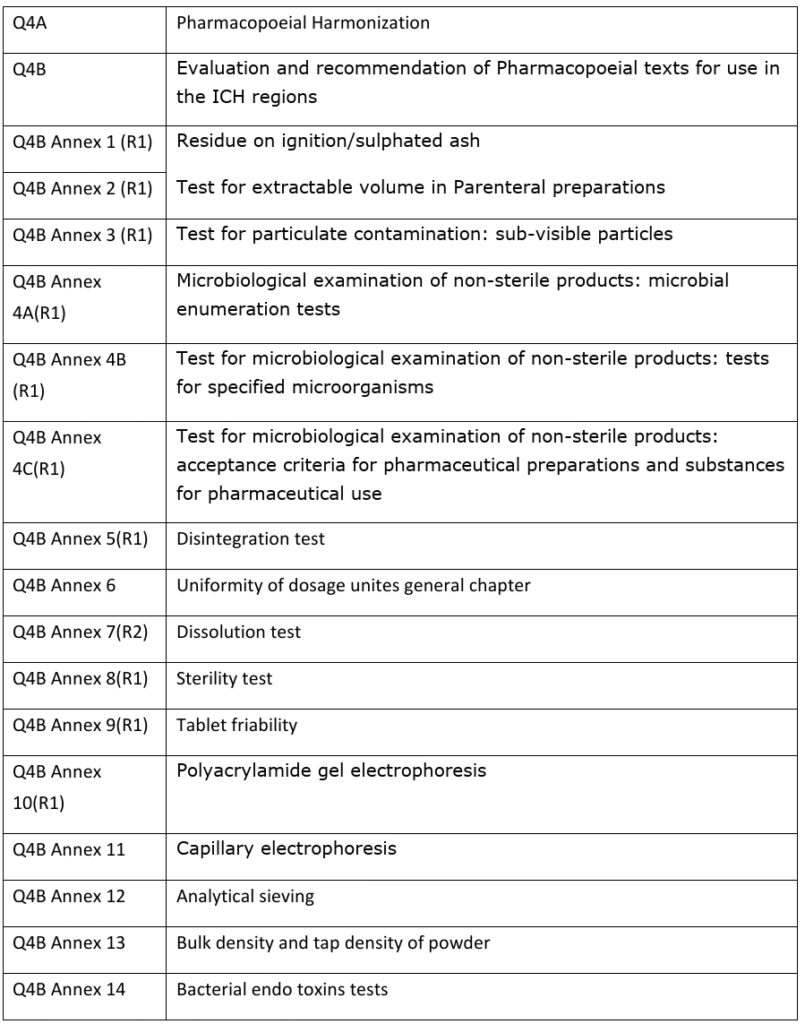
ICH Guidelines in Pharmaceutical (updated) » Pharmaguddu
08 - 11 February 2024 AMIA (American Medical Informatics Association) United States The SCRS Oncology Site Solutions Summit 10 - 11 April 2024 The Society for Clinical Research Sites (SCRS) United States PCC 2024 — Pharmaceutical Compliance Congress 16 - 18 April 2024 Informa United States SCDM 2024 EMEA Conference 17 - 19 April 2024

CCRB ICH GCP Knowledge Test
- New guidance on the electronic submission modalities of ICSRs under the new ICH-E2B(R3) format; - New guidance on the management of individual reports of off-label use, based on the Reflection Paper on Collecting and Reporting Information on Off-label Use in Pharmacovigilance (EMA/293194/2016), published for public consultation in 2016;

Good Clinical Practices Guideline ICHGCP Principles of GCP Hindi Pharmacovigilance
The ICH Harmonised Guideline was finalised under Step 4 in October 1994. This document gives standard definitions and terminology for key aspects of clinical safety reporting. It also gives guidance on mechanisms for handling expedited (rapid) reporting of adverse drug reactions in the investigational phase of drug development. Date of Step 4:

ICH Guidelines for Pharmacovigilance Organization and objectives of ICH Part 1 BPharm
Guideline on good pharmacovigilance practices (GVP) - Module VI EMA/542040/2014 (superseded version) Page 2/90 . VI.C.2.2.3.. in the ICH-E2A and ICH-E2D guidelines. 1. should also be adhered to ; they are included as well in this chapter. VI.A.2.1. Adverse reaction .

The Importance of ICH GCP CCRPS
This table lists ICH guidelines that have recently been finalised at ICH and are pending implementation or have either been implemented by Health Canada in the last 12 months.. Pharmacovigilance Planning. 2009/02/16. 09-103644-626.. Good Clinical Practice. 2019/04/03. 19-105427-311. E7: Guideline - Studies in Support of Special.

11. ROLE AND RESPONSIBILITIES OF CLINICAL TRIAL PERSONNEL AS PER ICH GCP PHARMD GURU
ICH E10 Choice of control group in clinical trials. ICH E11 (R1) step 5 guideline on clinical investigation of medicinal products in the pediatric population - Scientific guideline. ICH guideline E11A on pediatric extrapolation - Step 2b. ICH guideline E17 on general principles for planning and design of multi-regional clinical trials.

ICH Guidelines for Pharmacovigilance
1 ICH E6 Principles (Draft Version: March 2021) Clinical trials are a fundamental part of clinical research that support the development of new medicines or uses of existing medicines. Well designed and conducted clinical trials help answer key questions in health care and drug development.